Groups
Zinc Peptidase
CRISPR vectors for Lab research
Applied Biological Materials has CRISPR Clones for ZUP1 available from Gentaur at 125 euro
- ZUP1 CRISPR as ready-to-use vector or virus: Non-viral | Lenti- | Adeno- | AAV- | siRNA
- Cas9 Proteins for ZUP1, Cas9 Vector/Virus, Cas9-Expressing Cell Lines
- Elisa kits
- Antibodies
S-adenosylmethionine-binding proteins
Background
S-adenosyl-L-methionine is a source of diverse chemical groups used in biosynthesis and modification of virtually every class of biomolecules. The most notable reaction requiring S-adenosylmethionine, transfer of methyl group, is performed by a large class of enzymes, S-adenosylmethionine-dependent methyltransferases, which have been the focus of considerable structure-function studies. Evolutionary trajectories of these enzymes, and especially of other classes of S-adenosylmethionine-binding proteins, nevertheless, remain poorly understood. We addressed this issue by computational comparison of sequences and structures of various S-adenosylmethionine-binding proteins.
Results
- Two widespread folds, Rossmann fold and TIM barrel, have been repeatedly used in evolution for diverse types of S-adenosylmethionine conversion.
- There were also cases of recruitment of other relatively common folds for S-adenosylmethionine binding. Several classes of proteins have unique unrelated folds, specialized for just one type of chemistry and unified by the theme of internal domain duplications.
- In several cases, functional divergence is evident, when evolutionarily related enzymes have changed the mode of binding and the type of chemical transformation of S-adenosylmethionine. There are also instances of functional convergence, when biochemically similar processes are performed by drastically different classes of S-adenosylmethionine-binding proteins.
- Comparison of remote sequence similarities and analysis of phyletic patterns suggests that the last universal common ancestor of cellular life had between 10 and 20 S-adenosylmethionine-binding proteins from at least 5 fold classes, providing for S-adenosylmethionine formation, polyamine biosynthesis, and methylation of several substrates, including nucleic acids and peptide chain release factor.
Conclusion
We have observed several novel relationships between families that were not known to be related before, and defined 15 large superfamilies of SAM-binding proteins, at least 5 of which may have been represented in the last common ancestor.
Background
S-adenosylmethionine (SAM or AdoMet) is a conjugate of nucleotide adenosine and amino acid methionine, two ubiquitous biological compounds that almost certainly were present in the common ancestor of living cells and may have been found in the prebiotic environment on Earth, predating the origin of Life itself [1]. SAM is an essential metabolic intermediate in every studied cellular life form, and each cellular organism has several SAM-utilizing enzymes. One relatively well-understood biological role of SAM is to donate methyl groups for covalent modification of different substrates – from as simple as oxidized arsenic, chloride, bromide, and iodine ions [2-4], to as complex as rRNA, tRNA, and essential proteins, whose methylation status can serve as a regulatory signal for maturation and control interactions with other macromolecules ([5-7] and references therein).
Methyl transfer is but one of many biochemical processes requiring SAM. Enzymatic reactions that involve interaction of proteins with SAM or its structurally similar derivatives include transfer or methylene, aminoalkyl, ribosyl, and 5'deoxyadenosyl groups; formation of 5'deoxyadenosyl radical, which can be used as a redox intermediate in many reactions; SAM decarboxylation; and de novo synthesis of SAM from adenosine and methionine. There are also numerous interactions between SAM and non-enzymatic proteins, where SAM serves as a ligand triggering a regulatory change in the effector protein.
Despite the interest in this amazing variety of functions associated with SAM, and the known three-dimensional structures for representatives of almost every class of SAM-dependent enzymes, the structural, functional, and evolutionary relationships between the SAM-binding domains remain not well understood. Do all or some of the SAM-binding proteins share common evolutionary ancestry? How many distinct structural modes of interaction between SAM and protein are there? Is there strong or weak correlation between conservation of sequence and structure, the mode of SAM binding, and the chemical reaction facilitated by the enzyme? Finally, what may have been the repertoire of SAM-binding proteins in the ancestral organisms – in particular, in LUCA, the Last Universal Common Ancestor of the three present-day domains of Life – Bacteria, Archaea, and Eukarya?
We sought to address these questions by comparing sequences and structures of various groups of SAM-binding domains recognized in proteins. We describe several previously unsuspected relationships between some of such groups, predict novel members for many of them, and conclude that LUCA may have had more than a dozen of SAM-binding proteins, belonging to several distinct folds.
Results and discussion
- We have adopted the iterative comparison strategy, using the known or suspected SAM-binding protein domains as the queries in increasingly sensitive probabilistic methods of sequence modeling and database searching. In many cases, a SAM-binding part of the protein constitutes only part of the polypeptide chain. For example, methyltransferases typically consist of well-conserved SAM-binding portions and highly variable substrate-binding regions, sometimes further supplemented with portable domains also found in otherwise unrelated proteins, such as chromo domain interacting with methylated histone tails in eukaryotes, or PUA domain that probably interacts with RNA [8]. In this work, we are concerned with the protein moieties that bind SAM, so we neither examine these other domains, not consider methyltransferases that utilize other sources of methyl groups, like folate or methylcobalamin derivatives. We did not describe isoprenylcysteine carboxyl methyltransferase (ICMT), an integral endoplasmic reticulum membrane protein with unknown structure [9,10] (reviewed in ref. [11]).
- The phylogenetic relationships inside of several recognized groups of SAM-binding proteins, especially within Rossmann-fold SAM-dependent methyltransferases, have been reviewed recently [12,13]. Although we summarize and extend their observations, our main focus is on the analysis of more distant, previously unexamined, relationships.
- Versatile α/β architectures adapted for SAM binding
Sequence similarity

Figure: Comparison of SAM-binding proteins.
SAM-binding proteins with unique fold:2,5,19,20 (implying functional convergence in SAM-binding). Bottom-left = sequence similarity, top-right = structural similarity. Similar – red; not significant similarity – green; similarity not detected – black; unknown crystal structure – gray. Numbers represent COMPASS score for sequence similarities, or DALI Z-score for structural similarities. 1 - Rossmann fold methyltransferase, 2 - Met synthase activating domain, 3 - Methyltransferase class III, 4 - SPOUT methyltransferase, 5 - SET domain methyltransferase, 6 - methylene transferase, 7 - Nicotianamine synthase, 8 - Spermidine synthase, 9 - Spermine synthase (there were not enough sequences to make a good alignment, its sequence similarities should be very similar to these observed for spermine synthases), 10 - acalcynomycin-10-hydroxylase, 11 - mtTFB (mitochondrial Transcription Factor B), 12 - fluorinating enzyme (Streptomyces cattleya), 13 - guanine-n7-methyltransferase/guanyltransferase, 14 - QueA (tRNA ribosyltransferase-isomerase), 15 - SAM decarboxylase, 16 - SAM synthetase, 17 - ACC synthase, 18 - N-acyl-homoserine lactone synthase, 19 - Met repressor, 20 - CBS domain, 21 - DAPA synthase, 22 - SAM-dependent radical

Rossmanoids: ancient and ubiquitous SAM-dependent transferases
The majority of SAM-dependent methyltransferases belong to a large class of enzymes with the Rossmann-like fold, one of the more common arrangements of protein spatial structure, observed in dozens of diverse families of enzymes [14]. SAM-dependent methyltransferases are a large group of enzymes within the Rossmanoid class, and they account for a substantial fraction of all proteins in completely sequenced genomes; for example, with 1.7% of genes in Helicobacter pylori J99 coding for known or predicted SAM-dependent methyltransferases, this group makes the list of 10 most commonly used sequence and structure families in that species [15].
In the most basic arrangement, the Rossmann-like fold consists of alternating β-stranded and α-helical regions, with all strands forming a central relatively planar β-sheet, and helices filling two layers, one on each side of the plane. As with many other Rossmann-like folds, the N-terminal β-strand of methyltransferases is located in the middle of the sheet, and the strand topology is 3214576, with the 7th strand antiparallel to all other strands (Figure 1a). Yet another typical feature of Rossmanoid enzymes is that the functionally important, conserved residues are often located in the C-termini of the β-strands or in the adjoining loops [14]. Some methyltransferases conform to this plan quite well, with an occasional addition of an extra helix or a β-hairpin [16], or, rarely, deletion of one or both of strands 6 and 7 [17]. Most methyltransferases, however, contain additional domains appended or inserted into the basic Rossmann fold [16].
thumbnail Figure 1. Fold and topology of SAM binding proteins. Corresponding fragments of cartoon and topology representations of selected structures were rainbow colored from N-terminal (blue) to C-terminal (red) end. Less significant fragments of secondary structure were left white in topology diagrams. Reference to other representative structures are provided (in parentheses) as SCOP sunid numbers (i.e. ref. [139]). (a) 1ej0A, Rossmann-fold methyltransferase (53335 excluding: 102555, 69556, 69557, and 69560); (b) 1l1eA, cyclopropane fatty acid synthase (69560); (c) 1mjfA, spermidine synthase (69557); (d) 1vhvA, porphyrin C-methyltransferase (53789); (e) 1j6rB, Met synthase reactivation domain (methyltransferase; 56506); (f) 1mxiA, SPOUT methyltransferase (89629, 75218); (g) 1r30A, SAM dependent radical enzyme (102114); (h) 1mvhA, SET domain methyltransferase (82199); (i) 1rqpB, 5'-fluoro-5'-deoxyadenosine synthase (102521, 101851); (j) 1m7y, 1-aminocyclopropane-1-carboxylate (ACC) synthase (53441, 64130, and similar but different: 53439); (k) 1vkyA, tRNA-ribosyl transferase-isomerase (111338); (l) 1o9tB, methionine adenosyltransferase (55972); (m) 1cmc, MetJ – methionine repressor (dimer; 100972); (n) 1msvA, SAM decarboxylase (56275); (o) 1pbjA, CBS domain (dimer; 54630 – not all CBS domains bind SAM); (p) 1kzfA, acyl-homoserine lactone synthase (75508).
Notwithstanding the insertions of additional domains and structural elaborations, comparative sequence analysis of the Rossmann-fold methyltransferases identifies the set of five highly conserved regions of the SAM-binding region, each centered on one or more nearly-invariant residues (Figure 2). They correspond to motifs I-V from motifs initially proposed for DNA:m5C MTases by Posfai et al. [18] (reviewed in refs. [12,19]), but some of the conserved residues highlighted in this work have not been pointed out before (see below). Each motif has a clear counterpart at the structural level. Five motifs are arranged in the same linear order in almost all known methyltransferases, with a notable exception of several groups of DNA- and RNA-methyltransferases, where circular permutation of the sequence results in a main chain fission after motif II, while the spatial structure of the domain and mode of SAM binding remain virtually unperturbed (discussed in more detail by Bujnicki [20]).
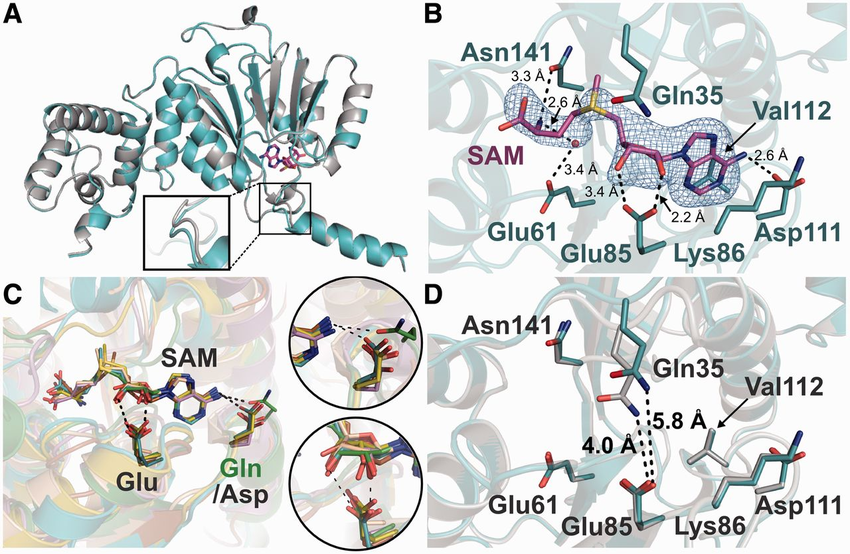
Figure 2. Multiple sequence alignment of Rossmann-fold methyltransferases and nicotianamine synthase. Sequences are denoted by NCBI gi number, short protein name (when available, otherwise COG/KOG/Pfam number was used), and abbreviated species name (as in UniProt Knowledgebase [140]). Nicotianamine synthase is marked by a blue box. Conserved motifs are labeled above the alignment. Conserved residues are marked by asterisk. Consensus positions of the secondary structure elements are shown above the alignment. Numbers in parentheses indicate number of residues omitted for clarity. Residues are highlighted according to the amino acid properties. Gray shading indicates conservation of single residue. Red font indicates conservation of acidic residues (D, E). Cyan font indicates conservation of Ser/Thr (S, T). Blue gray font with yellow shading indicates conservation of aliphatic residues (I, L, V). Dark blue font indicates conservation of basic residues (H, K, and R). Green font indicates conservation of tiny residues (A, G, and S). Blue font with yellow shading indicates conservation of aromatic residues (F, H, W, and Y). Pink font indicates conservation of charged residues (D, E, H, K, and R). Dark green font indicates conservation of small residues (A, C, D, G, N, P, S, T, and V). Bright blue font indicates conservation of polar residues (C, D, E, H, K, N, Q, R, S, and T). Blue font with pale yellow shading indicates conservation of big residues (E, F, H, I, K, L, M, Q, R, W, and Y). Black font with yellow shading indicates conservation of hydrophobic residues (A, C, F, G, H, I, L, M, T, V, W, and Y).
The first conserved sequence block (Motif I) includes in its C-terminal part the consensus GxGxG, considered the hallmark SAM-binding site of the Rossmann-fold SAM-dependent methyltransferases. None of three glycine residues is universally conserved, but the replacements are typically by the residues with small side chains, or with propensity of bending the main chain. This agrees with the structure data, indicating that the consensus is located in a loop connecting the first β-strand and the α-helix in the Rossmann fold core. The complete β-strand and part of the preceding loop are also part of Motif I. In the middle of β-strand 1, there is an exceptionally well conserved acidic residue (D or E); one or more conserved positively charged residues are found close to the N-terminus of this strand (Figure 2).
Motif II encompasses β-strand 2 and adjoining turn. A partially conserved acidic residue is common at the C terminus of this strand. Motif III corresponds to β-strand 3, located at the edge of the β-sheet in the Rossmann fold. An acidic residue is partially conserved close to the C-terminus of this strand, too. Whenever the substrate (SAM), its analogs, or reaction product (SAH) are co-crystallized, they are found close to the invariant residues in Motifs I-III (Figure 2 and see below).
Motif IV consists of β-strand 4 and the flanking loops. In this motif again, there is a well-preserved D/E/N residue, located at the extreme N-terminus of the strand, i.e. at the side of the fold that is not involved in substrate binding. Motif V corresponds to the helix following the strand with motif IV. In some Rossmann-fold methyltransferases, it serves as a scaffold for large hydrophobic or aromatic side chains that stabilize the adenine moiety of AdoMet, but it many cases it has been shows these residues are not essential for the MTase activity [21]. Finally, Motif VI corresponds to Strand 5 of the β-sheet, and the preceding tight turn with a nearly-invariant glycine residue.
Several residues from Motifs I-V are known to make direct contact with SAM. In particular, one or more residues in "GxGxG" loop are in contact with the carboxypropyl moiety of SAM, while conserved acidic residue in Motif II forms hydrogen bonds with the ribose hydroxyls (Figure 2; ref. [22]). Variable residues at the C-termini of strand 2 (Motif II) and conserved acidic residue in Strand 3 (Motif III) interact with the nitrous base, while variable residues C-terminal to Strand 4 (Motif IV) appear to contact the amino and sulfonium groups of the methionine moiety of SAM [22]. Residues from motif IV, VI, VIII, and sometimes X are associated with the catalytic pocket, where residues from motif V and VII are important mostly for the structural stability [19,23].
The roles of other conserved residues in SAM-dependent methyltransferases are less well understood. Near-omnipresence of the D/E residue in Motif I suggests that it has an important role. It has been noted [24], that in FtsJ RNA methyltransferase this residue coordinates SAM through a water molecule. In fact, in all 3-D structures of methyltransferases where solvent molecules are present (i.e. PDB structure 1EJ0, 1KYW, and 2ADM), the oxygen atoms in the carboxyl group of this D/E residue make direct contacts with two water molecules, one of which is capable of forming a hydrogen bond with the side chain of methionine moiety of SAM. In some ribose 2'-O-MTases, D/E amino acid conserved in motif I is substituted by tyrosine (Figure 2 and ref. [25]), and it has been proposed that this residue could be used to directly (not via the water molecule) coordinate the amino-carboxyl end of SAM (J.M. Bujnicki, personal communication).
These observations are of interest for understanding the mechanism of methyl transfer by Rossmann-fold methyltransferases. Two best-studied groups of transferases that have Rossmann-like fold and use a nucleotide derivative as a cofactor, namely ATPase-like kinases and nucleoside diphospho-sugar transferases, appear to require a divalent metal cation for polarization of water molecule that can then attack a scissile phosphoester bond [26-29]. Methyltransferases, on the other hand, need to work on a C-S+ bond in SAM, but do not seem to have any metal ion bound in the appropriate position (even though divalent cations have been included in some crystallization media). The proposals for reaction mechanisms of different classes of SAM-dependent methyltransferases include nucleophilic catalysis, with the identity of nucleophile ranging from moderately conserved residues scattered across the SAM-binding domain to bound water molecule [30], as well as SN2 reaction, which would require initiation by concerted action of several side chains, or, perhaps, by the amino group of the substrate itself [31]. The highly conserved D/E residue in motif I may, however, provide a unifying theme in the catalysis, by polarizing a water molecule that is close to the methyl group of SAM. The water molecule could either serve as a nucleophile, or aid bond displacement between the sulfonium ion and methyl group in some other way.
Finally, we noticed that the conserved basic residue at the beginning of Motif I and nearly-invariant acidic residue at the beginning of motif IV are typically located within a short distance (3Å or less) of each other, potentially forming a salt bridge that may be important for locking other elements of the Rossmann fold in place (Figure 2).
Rossmann-fold SAM-binding proteins that do not have methyltransferase activity
I. Methylene transferases
Formation of the cyclopropane ring in unsaturated fatty acids by cyclopropane fatty acid synthase [EC: 2.1.1.79] has been studied extensively in bacteria. The reaction involves transfer of a methylene group from SAM to the double bond of an unsaturated acyl chain [13,32].
Crystal structures of mycolic acid cyclopropane synthases CmaA1, CmaA2, PcaA, and MmaA2 have fold similar to Rossmann-fold methyltransferases (Figure 1b), with the conserved position of SAM and very similar pattern of interactions with the cofactor [32]. A hallmark of methylene transferases is the presence of the carbonate ion (CO3 2-) at the active center, which probably enables the formation of carbocation intermediate required for completion of the reaction (Figure 3) and ref. [32]. Conserved residues involved in carbonate ion binding (Cys35/Ser, His167/Gln, and Tyr232/Phe – numbered as in PDB structure 1L1E) appear to distinguish methylene transferases from Rossmann-fold methyltransferases.
thumbnailFigure 3. Structural alignment of cyclopropane fatty acid synthase and Rossmann-fold methyltransferase. Red color – YecO methyltransferase (PDB structure 1IM8:A) Yellow color – ligand in YecO methyltransferase. Green color – mycolic acid cyclopropane synthase CmaA2 (PDB structure 1KPI:A). Blue color – three ligands in CmaA2 (PDB structure 1KPI:A). Motifs I-VI conserved in Rossmann-fold methyltransferases are labeled above the alignment.
II. Amino alkyl transferases: nicotianamine synthase and spermidine synthase
Nicotianamine synthase (S-adenosyl-L-methionine: S-adenosyl-L-methionine: S-adenosyl-L-methionine 3-amino-3-carboxypropyltransferase, EC: 2.5.1.43) catalyses direct condensation of three molecules of SAM, followed by the formation of an acetidine ring, to yield one molecule of nicotianamine, a chelator of various transition metals ubiquitously present in higher plants. In graminaceous plants, nicotianamine is the precursor of phytosiderophores that are secreted from the roots to solubilize ferric iron in the soil. Reduced levels of endogenous nicotianamine affect the development of plant reproductive organs and seeds' maturation [33].
Protein structure of nicotianamine synthase is not known, but sequence similarity searches indicate a significant similarity between nicotianamine synthase and Rossmann-fold methyltransferases. A PSI-BLAST search, using with Arabidopsis thaliana NAS [GenBank:O80483] as a query, matched Pseudomonas syringae ubiE/COQ5 methyltransferase [GenBank:YP_233497] with E-value = 1e-21 and score = 105 at 4th iteration; PDBBLAST top match is to (N5)-Glutamine Methyltransferase [PDB:1T43]. Similarity is the highest in conserved motifs II-IV and VI (Figure 2), and motif I is also conserved, in a modified form (F-x-G-S-G-P-x-P). Interestingly, related sequences with the same modification of Motif 1 are found in archaea (Methanothermobacter thermautotrophicus, [GenBank:NP_275817], bacteria (Pseudomonas aeruginosa, [GenBank:NP_253523], and fungi (Neurospora crassa, [GenBank:XP_330777]. The replacement of the conserved D/E/N residue in motif I (see above) may partially explain the change in the functional group that is transferred from SAM: unlike the related Rossmann-fold methyltransferases, nicotianamine synthases lack negatively charged residue in Motif I, so the aminoalkyl moiety of SAM is not bridged to the enzyme by a water molecule and is free to leave in the course of the reaction.
Spermidine synthase (EC: 2.5.1.16) catalyzes the transfer of the aminopropyl group from decarboxylated SAM to putrescine to form spermidine. Putrescine, spermidine and spermine, formed from spermidine, are polyamines essential for the regulation of cell proliferation and differentiation in most species, and gram-negative bacteria outer membrane permeability in response to the acid stress [34,35]. Spermidine synthase is an oligomeric enzyme, each monomer consisting of a C-terminal domain with a Rossmann-like fold and an N-terminal tetramerization β-stranded domain [36].
Spermidine synthase has very high sequence similarity (approx. 70% identity) to putrescine N-methyltransferase. It has been shown that aminopropyl moiety of spermidine synthase inhibitor – AdoDATO (a compound containing both substrate and product moieties) binds in a similar orientation to the homologous part of SAM in Rossmann-fold methyltransferases. However, the binding site in spermidine synthase contains invariant residue Asp101 (PDB structure 1JQ3), located in the middle of glycine-rich loop (corresponding to motif I of Rossmann-fold methyltransferases) making binding cavity too small to accommodate the carboxyl group of SAM, that prevents SAM binding and enables specific binding of decarboxylated SAM [36]. The polyamine moiety of AdoDATO is oriented toward putrescine binding cleft. Invariant Asp170 (corresponding to D/N residue located at the end of β-sheet in motif-IV of Rossmann-fold methyltransferases) is most likely candidate to deprotonate putrescine, enabling it to perform a nucleophilic attack on methylene carbon of decarboxylated SAM [36].
III. Acalcynomycin-10-hydroxylase
Aclacinomycin 10-hydroxylase RdmB is a methyltransferase homolog that catalyses a SAM-dependent hydroxylation of the C-10 carbon atom of 15-demethoxy-ε-rhodomycin, a step in the biosynthesis of the polyketide antibiotic β-rhodomycin. In order to activate molecular oxygen, the enzyme uses SAM instead of cofactors usually associated with hydroxylase activity, such as flavins, 2-oxoglutarate, or metal ions. It has been proposed that positive charge of the SAM cofactor plays a role in delocalisation of electrons into the anthraquinone core of the substrate [37]. The C-terminal domain of RdmB has an α/β Rossmann-like fold, and contains the conserved signature DLGGGxG in motif I.
The enzyme lacks methyltransferase activity due to the positioning of SAM in which the methyl group points in a direction unfavorable for a SN2 type methyl transfer to the substrate [37]. The difference in SAM-substrate positioning is not well understood, but might be related to differential interactions between SAM binding C-terminal domain and substrate binding N-terminal domain or differences in the conserved loop (amino acids 292–298) [37].
Non-catalytic Rossmannoids
The lack of detectable SAM binding motifs in several Rossmann-fold methyltransferases suggests that they may be recruited for a new function. For example, the three-dimensional structure of sc-mtTFB (Saccharomyces cerevisiae mitochondrial transcription factor B) bears strong resemblance to ribosomal RNA adenine dimethylases (i.e. KsgA and ErmC'). However, several residues required for interaction with SAM are not conserved in sc-mtTFB; in particular, the glycine-rich loop (motif I) contains bulky Tyr residue, and motif IV is poorly conserved.
Human co-orthologs of sc-mtTFB (h-mtTFB1 and h-mtTFB2) have rRNA N6-adenine methyltransferase activity (in an Escherichia. coli assay), but mutational analysis of h-mtTFB1 indicates that this activity is not required for transcriptional activation [38].
In Gcd10p/Gcd14p complex – tRNA(1-methyladenosine) methyltransferase of S. cerevisiae, the lack of SAM binding was observed in Gcd10p that directs binding of tRNA, where Gcd14p binds the required cofactor S-adenosylmethionine [39,40].
In another case, Kar4p (pheromone induced, karyogamy-specific transcription factor) does not bind SAM, where similar (circularly permuted) Ime4p is SAM-binding methyltransferase [41].
In bacterial rRNA:m2G methyltransferases RsmC and RsmD the inactivated domain and the catalytic domain are fused together in one polypeptide [42].
The de novo methyltransferase-like protein, DNMT3L, is required for methylation of imprinted genes in germ cells. Although enzymatically inactive, human DNMT3L accelerates DNA and SAM binding to de novo DNA methyltransferases [43-45].
Rossmann-like domain of bacterial fluorinating enzyme
Actinomycete Streptomyces cattleya is able to produce C-F bonds using inorganic fluoride. The fluorinating activity requires SAM, and the primary product of the reaction is SAM derivative, 5'-fluoro-5'-deoxyadenosine [46]. The protein, 5'-fluoro-5'-deoxyadenosine synthase FlA, belongs to a conserved sequence family represented in most archaea and in a subset of bacteria [46].
The structure of FlA consists of two domains – a larger N-terminal domain with α/β fold, and a smaller C-terminal β-barrel. Both domains interact with SAM and with reaction products [46]. FlA is a hexamer in solution and trimer in crystal, and three SAM molecules are bound by a trimer, between the N-terminal domain of one subunit and the C-terminal domain of the adjoining subunit. This arrangement, however, appears to be dependent on a long (24 amino acids) loop in the N-terminal domain, which is missing from the closely related sequences in all other species. On the other hand, the linker connecting two domains in a monomer is long enough to allow significant domain motions, and it is plausible that two domains may interact in other oligomeric arrangements and perhaps even within a monomer. Therefore, we speculate that SAM binding by FlA-like proteins from other species may occur in the crevice formed by the N- and C-terminal domains of the same molecule, and the following discussion does not consider the oligomerization state.
The N-terminal domain makes contacts mostly with methionine, ribose, and fluoride ion, and C-terminal domain contacts methionine and adenine ring. The α/β N-terminal domain exhibits several features similar to other enzymatic domains with Rossmann-like topology, namely: three-layer α/β/α architecture; the planar central, mostly parallel β-sheet filling "inside-out" (strand topology 2135467), and concentration of the substrate-binding and catalytic residues in the loops following the C-termini of strands. More specifically, the loop after strand 1 contains Asp16 (numbered as in PDB structure 1RQP) hydrogen-bonded to both hydroxyls of ribose, Leu17 that may be involved in water-mediated interaction with methionine amino group, Asp21 and Ser23, both of which can form hydrogen bonds with the same amino group. Loop following strand 2 contains Trp50 that is able to contact one ribose hydroxyl and perhaps to have Van der Waals interactions with the adenine ring. Loop between strands 3 and 4 contain two ribose interactors, Thr76 and Tyr77. Loop after strand 6 hosts Thr155, which is part of hydrogen-bond network linking two domains via two water molecules and methionine carboxyl group, as well as catalytic Ser158 that is expected to make two polar contacts with deprotonated fluoride ion [46]. Although most of these interactions are provided by residues located in loops at the edge of β-sheet, there is no specific sequence similarity between Rossmann-like domain in fluorination enzyme and in Rossmann-fold methyltransferases. There is also no similarity to other SAM-utilizing enzymes.
Rossmann-like fold in SPOUT methyltransferases?
A distinct superfamily of SAM-dependent methyltransferases, SPOUT, which includes families specified by bacterial SpoU, TrmD, and TrmH, proteins, as well as many uncharacterized proteins in all three domains of Life, have been shown to share a set of conserved sequence elements and an α/β-type fold [47]. Trm10, a recently characterized tRNA m1G9 methyltransferase, is also predicted to have this fold [48,49]. All experimentally characterized members of this large superfamily are DNA or RNA methyltransferases. A unique structural feature of this α/β fold is a trefoil knot of two crossing loops in the C-terminal region [47].
Several hallmarks of Rossmann-like structure are evident in the SPOUT fold. There are three main layers, with a central β-sheet sandwiched between two helical layers; the β-sheet is formed "inside-out", with the first and one of the last strands in the center of the sheet; and the SAM ligand interacts mostly with the conserved residues located in the loops at the C-termini of β-strands [50]. There is, however, no sequence similarity between SPOUT-fold methyltransferase and any other Rossmann-fold SAM-binding protein.
Structural similarity between SPOUT-fold and Rossmann-fold methyltransferases (i.e. DALI Z-score = 3.1 for 88 aa with RMSD = 3.7 and sequence identity = 17% for PDB structures 1QAO and 1MXI) is confined mainly to the N-terminal half of those folds. There is no similarity in the C-terminal part, where strands 4 and 5 are rearranged.
SAM binding inside and outside of β-barrels
SAM-radical enzymes: recruitment of ancient enzymatic TIM barrel
A (β/α)8 fold, also known as triose phosphate isomerase (TIM)-like barrel, is one of the largest classes of protein structures, exceeding even Rossmann-like fold in omnipresence and versatility [51]. Most of TIM-barrel proteins are enzymes, belonging to almost all of the major EC classes [52]. A well-known version of a TIM barrel is a (β/α)6 "semi-barrel," in which the inner layer of slanted β-strands does not form a complete cylinder, but has a lateral opening (PDB structure 1OLT).
Recently, several structures of proteins from a large sequence family of "SAM radical" enzymes (ref. [53]; Figure 1g) have been determined, and it became evident that members of this family have (β/α)6 and (β/α)8 folds. SAM-radical enzymes utilize non-covalently linked Fe-S cluster and a SAM molecule, in a reductive cleavage reaction that produces methionine and 5'-deoxyadenosyl radical, that can be used to generate further glycyl or thiyl radicals on the same protein molecule or on a coupled enzyme [54]. It has been noted that SAM-radical sequence family is very large, diverse, but can be recognized by a hallmark CxxxCxxC signature close to the N-terminus, followed by another conserved "GG" motif [55].
We detected more than 2000 non-redundant sequences from SAM-radical family in the sequence databases. Interestingly, when the region containing the three characteristic cysteines was deleted from the queries, the searches resulted in almost the same collection of sequences as with full-length domain, indicating strong evolutionary signal along the stretch of 200–250 residues to the C-terminal side of the CxxxCxxC signature. Multiple alignment of many representative sequences identified four regions with high sequence similarity and three weaker conserved motifs (Figure 4). Comparison of the alignment with the known structures of biotin synthase (PDB structure 1R30), coproporphyrinogen III oxidase (PDB structure 1OLT) and molybdenum cofactor biosynthesis enzyme MoaA (PDB structure 1TV7) suggests structural and functional correlates for these regions and for the most conserved residues within them. The best-conserved motifs correspond to the β-strands of the inner barrel and their C-terminal loops, while the regions of additional partial conservation correspond to the outer-shell α-helices.
thumbnailFigure 4. Multiple sequence alignment of SAM-dependent radical enzymes. Sequences are denoted by NCBI gi number, conserved domain name (as in NCBI CDD database [141]), short protein name (if available), and abbreviated species name. Secondary structure elements extracted from PDB structure 1OLT coordinates are shown above the alignment. Residues are highlighted according to the amino acid properties with designations as in Figure 2.
Motif 1 includes the most N-terminal β-strand in the (β/α)6 barrel (strand 1). Three invariant cysteine residues in the adjoining loop (Figure 4) coordinate the [Fe-S]4 cluster, which is present in a similar configuration in all protein structures resolved thus far. One iron atom has no contact with the cysteine side chains, and is instead ligated by the N and O atoms from the amino- and carboxy groups of SAM. Also highly conserved is aromatic or heterocyclic residue (Y, F, or H) preceding the last of the three cysteines; the main chain of this residue seems to form a hydrogen bond with the adenosyl moiety of SAM, but the significance of the side chain conservation is unclear; perhaps it contributes to the non-polar milieu of the bound Fe-S cofactor, preserving it from oxidation.
The second prominent motif does not contain any invariant amino acids, but includes several residues with small side chains, most often two or three glycines in a row (Figure 4). This motif corresponds to the second strand in the barrel and the tight turn after the strand. The main chain of this turn is within a contact distance from the amino group of the methionine part of SAM. The third motif also corresponds to the strand-turn structure. A signature T/S-N-G that follows strand 3 is well conserved; as a rule, residues in this turn form hydrogen bonds with the carboxyl group of methionine in SAM. The fourth motif consists of strand 4 and the loop with a highly conserved acidic or amide residue (D, E, N, or Q). Typically, this residue is within hydrogen-bonding range from both 2'- and 3'hydroxyl groups of the ribose ring of SAM. The strands 5 and 6 followed by loops provide one or more residues that form hydrogen bonds with the amino group of adenosyl; however, sequence conservation in these regions is moderate.
The heterogeneity of the SAM radical protein superfamily is most pronounced in their C-terminal regions, which are responsible for binding of substrates and auxiliary cofactors. On the other hand, the structure and sequence of the N-terminal, SAM-binding region of SAM-radical proteins is well conserved, analogously to the Rossmann-fold methyltransferases. The SAM-binding region is essentially an incomplete (β/α)6 "semi-barrel," which is typically modified by evolutionarily diverse elements (commonly consisting of α-helices, but sometimes also containing β-hairpins or small sheets) that serve substrate-binding and regulatory roles.
Rossmann folds and TIM-barrels in fact have quite similar β/α architectures. This becomes especially evident in the case of incomplete barrels. The primary difference is lack of one α-layer in TIM-barrels, and correlated changes in sheet curvature and strand orientation. The two classes of SAM-binding enzymes both use loops between strands and helices to interact with various moieties of relatively extended SAM molecules, but the details of this interaction are quite different (see below).
TIM barrel-like catalytic domain in QueA?
Queuosine is a hyper-modified nucleoside in bacterial and eukaryotic tRNAs, produced by a multi-step enzymatic pathway that includes a transfer, with simultaneous isomerization, of ribose moiety from SAM to a modified base in tRNA, called 7-(aminomethyl)-7-deazaguanosine, or preQ1. This step is performed by QueA protein, an S-adenosylmethionine:tRNA ribosyltransferase-isomerase. QueA homologs are found in most bacteria, but their sequence is not strongly similar to any other protein family, and high-resolution structure of QueA in complex with SAM is unavailable. We interrogated the fold recognition meta-servers with individual QueA sequences and with a probabilistic model of aligned QueA homologs. The highest 3D-Jury consensus score (69 units, indicating the upper level of the "gray zone" of provocative, if statistically insignificant, sequence similarities [56]) was to pyruvate kinases, a distinct class of proteins with three structural domains. The C-terminal, regulatory domain of pyruvate kinases has no counterpart in QueA. The other two domains are arranged in such a way that a smaller, β-barrel domain is inserted into the larger, α/β TIM-barrel domain but folds independently. Similar arrangement of two domains is predicted for QueA.
When this manuscript was under preparation, the structure of QueA from Thermotoga maritima was resolved (PDB structure 1VKY). In agreement with the fold recognition data, it shows an α/β domain with insertion of independently folding β-barrel (Figure 1k). The structure of the α/β domain shows one β-sheet, with preponderance of α-helices on one side (Figure 1k). This structure resembles a semi-barrel, given a strongly curved β-sheet, relative absence of α-helices on the concave side, and a lid-like irregular arrangement of elements that covers the cavity. There is an unresolved protein segment of 32 residues, which should be located close to the inner β-layer of the semi-barrel, and may in fact extend its wall. An unresolved ligand is placed in the proximity of the C-termini of several β-sheets, and if this is in fact SAM, its binding mode would be similar to what is observed in other SAM-binding proteins (see below).
β-barrels in QueA and fluorination enzyme
Both QueA and fluorination enzyme structures show fusions of a larger α/β domain and a smaller all-β domain with barrel-like topology. The role of all-β domain in QueA is unclear, but it is not very likely to be involved in interaction with SAM. In contrast, the β-barrel domain in FlA (which, in fact, is more similar to "smashed β-can," with one side caved in, producing a double-concave surface) makes many contacts with the ligand. Proceeding from the N- to C-terminus, the Asp210-His211 (as in PDB structure 1RQP) dipeptide in the loop after the first strand bonds with the amino group of methionine; Asn215 bonds with the amino group of adenine; Ser269 and Arg270 after strand 5 can form 4 hydrogen bonds altogether, all with the carboxyl group of methionine; and at the C-terminus of strand 5, Arg277 and Ala279 provide additional interactions with adenine. While the catalytic mechanism of FlA is dependent on correct positioning of the fluoride atom with regards to ribose, which is mediated by Ser158 in the Rossmann-like domain (see above and reference [46]), the β-barrel domain appears to be essential for correct orientation of SAM, which serves as fluoride acceptor.
Between the sheets: double-β SAM-binding folds with a common theme of internal domain duplication
Decarboxylase
S-adenosylmethionine decarboxylase (EC: 4.1.1.50, SAMDC) is a key enzyme in spermidine and spermine biosynthesis. It produces decarboxylated SAM (dcSAM), which then donates aminopropyl group to putrescine or spermidine, two essential intermediates in polyamine biosynthesis. Because polyamines link diverse pathways in cellular metabolic networks, and because chemical inhibitors of SAMDC display potent antitumor and anti-parasite activities [57,58], structure-function relationships of SAMDC are of considerable interest.
SAMDC activities have been purified from all three domains of Life (bacterium E. coli, archaea Methanococcus jannaschii, and several eukaryotes), and certain common features of the enzymes have been noticed. All three enzymes are processed in vivo, forming a small subunit derived from the N-terminus and a large subunit accounting for the rest of the molecule; the N-termini of all large subunits contain a pyruvoyl group, produced from a serine residue by autoprocessing and required for the formation of the Schiff base during catalysis. All studied enzymes form multimers from the heterodimers of large and small subunits. There are also differences among bacterial, archaeal and eukaryotic SAMDC: the length of the precursor proteins in different species varies from 105 to 460 amino acids; mammalian enzymes require putrescine for full activity, E. coli enzyme requires Mg2+ cation, while archaeal and plant enzymes apparently do not require those factors. The bacterial enzyme is a tetramer of heterodimers, while eukaryal and archaeal enzymes are homodimerized heterodimers.
Comparative sequence analysis has revealed statistically significant sequence similarity between archaeal and bacterial SAMDC [59]. Multiple alignment of these two classes of SAMDC spans the complete length of the shorter (ca. 120 aa) archaeal enzymes, and also suggests that there are two types of bacterial enzymes – some are about the same size as archaeal SAMDC, and some are longer and phylogenetically distinct (reference [60], Figure 5a, and unpublished observations). No sequence similarity has been reported between these enzymes and eukaryotic SAMDC.
thumbnailFigure 5. Multiple sequence alignment of SAM decarboxylases. Sequences are denoted by gene and species name. A: Decarboxylase alignment – the residues underlined on the top line are those involved in SAM-binding, enzyme self-processing, and catalysis. Residues are highlighted according to the amino acid properties with designations as in Figure 2. B: Superimposition of the conserved residues in SAMdc from T. maritima (PDB structure 1TLU), human (PDB structure 1I7B) and potato (PDB structure 1MHM) are shown on cartoon representation of T. maritima structure (gray). Blue – Ser; Red – Ser and Ser converted into pyruvoyl group (or pyruvoyl group with covalently bound S-adenosylmethionine methyl ester); Orange – His; Cyan – Cys.
High-resolution structures of eukaryotic SAMDC from humans and plants in complex with substrate analogs and various inhibitors have been reported. The heterodimer folds as a sandwich of two β-sheets between α-helical regions, where the smaller subunit forms a half of one β-sheet, and the larger subunit completes this sheet and accounts for all the strands in the other sheet. The arrangement is unique among the known protein folds, but visual inspection and superposition of the two α-β halves of the molecule revealed their remarkable similarity and suggested the hypothesis of internal duplication [61,62]. The evolutionary origin and catalytic mechanism of prokaryotic SAMDC remained unclear.
Searches of sequence databases with the PSI-BLAST program and more involved probabilistic models of aligned SAMDC enzymes confirmed statistically significant sequence similarity between archaeal and bacterial enzymes, and also, intriguingly, produced several statistically insignificant local matches to one-half of eukaryotic SAMDC sandwich, in the area corresponding to the β-strand 12 in the three-dimensional structure. This strand is positioned next to the active center of the enzyme, and contains residues important for catalysis and/or binding of the substrate (see below). Because both these residues appeared to be preserved in the BLAST output, we sought better statistical validation of this similarity using Metaserver [56]. When SAMDC homolog from archaea Archaeoglobus fulgidus was used as a query, the highest 3D-Jury consensus score (46–60) was reported to the set of the eukaryotic SAMDC structures; this score is at the top of the zone with borderline significance, where most of the non-trivial similarities are discovered [56]. The first false positive (bacterial luciferase) was associated with the sharp drop in the 3D-Jury scores (14.5).
Almost complete archaeal sequence can be aligned to the half of eukaryotic template, with just one short gap. Conversely, the aligned region of the template corresponds almost precisely to the C-terminal half of the double sandwich. We conclude that the archaeal enzyme may resemble a half of the eukaryotic SAMDC fold and may be directly related to the pre-duplication ancestor of that fold. Multiple sequence alignment of archaeal, bacterial, and eukaryotic enzymes strongly reinforces these observations (Figure 5a). The C-terminal halves of eukaryotic enzymes could be aligned to prokaryotic homologs directly and unequivocally; the structurally similar N-terminal halves had to be superimposed using the knowledge of secondary structure and information about a few conserved residues.
The functional and evolutionary implications of the alignment are provocative. In mammalian enzymes, SAM decarboxylase is active as a dimer in which each protomer contains one large and one small subunit, and each of the two halves of the sandwich contributes several residues to binding the substrate and actually performing the catalysis. In particular, Ser residue in β-strand 4 of the eukaryotic enzymes, which is converted into catalytic pyruvoyl group, appears to be within a short distance of the carboxyl group of SAM forming a Schiff base adduct with it. Before product release, carbon of decarboxylated SAM is protonated by adjacent Cys (Figure 5b). This protonation regenerate the pyruvoyl group [63]. Also close to the active site is the side chain of the histidine residue in strand 12, which is believed to be responsible for abstraction of a proton from the α-carbon of the catalytic serine during proenzyme processing [64].
Two acidic residues contribute to binding of SAM: glutamic acid at the C-terminus of strand 3 contacts the base, and another glutamate, at the C-terminus of strand 12, interacts with both hydroxyl groups of the ribose ring. All these residues are conserved in eukaryotic SAMDCs – some in the N-terminal half of the sandwich, and others in the structurally equivalent C-terminal half. Interestingly, in archaeal and most bacterial enzymes, the pattern of conservation of these residues appears to be the union of conserved elements in the two halves of eukaryotic enzymes (Figure 5a), as if the bacto-archaeal enzyme is a homolog of one half of the eukaryotic enzyme, and the β-sandwich in the holoenzyme are made of two identical molecules.
When this manuscript was in preparation, the structure of ligand-free holoenzyme from bacterium T. maritima was deposited in the database (PDB structure 1TMI). Analysis of this structure confirms this sequence-based prediction and suggests that the bacto-archaeal form is ancestral, and the eukaryotic form has been derived from it by domain duplication/fusion, followed by functional specialization of two halves (most notably, by mutating the C-half of the enzyme so that it no longer undergoes autoproteolysis – Figure 5b).
SET domain
Discovered as conserved domain shared by chromatin remodeling proteins Su(var)3–9, E(Z) (short for Enhancer of Zeste) and Trithorax, SET domains turned out to be a distinct class of SAM-dependent methyltransferases. All studied SET methyltransferases transfer methyl group to lysine within various nuclear proteins involved in chromatin function and regulation of transcription, such as histones, TAF10, tumor suppressor p53, but also in such diverse proteins as Rubisco and cytochrome C [65-68].
In SET-domain methyltransferases amine of the substrate lysine residue access the methyl donor (SAM) through a narrow channel connecting the substrate and SAM binding surfaces [69]. SAM binding site and the catalytic center of all studied SET domains seem to be constructed on the unusual but conserved, all-β, knot-like structure [70]. Adenosyl moiety of SAM interacts directly and indirectly, through water, with conserved histidine (PDB structure: 1O9S-His297; 1P0Y-His243). This histidine may serve as a proton acceptor for the hydroxyl group of invariant Tyr (PDB structure 1O9S-Tyr335). The -OH of this Tyr is within 4 Å of the presumptive location of the substrate Lys Nζ, and may be involved in Lys side chain deprotonation (deprotonated Lys is presumed to make a nucleophilic attack on the SAM methyl group). Positively charged amino nitrogen from SAM hydrogen bonds with the side chain of invariant asparagine (PDB structure 1O9S-Asn296). This interaction may contribute to the compact conformation of the SAM molecule.
Phylogenetic analysis of the SET domain suggests that it is an evolutionary innovation in the eukaryotic lineage (with secondary lateral transfer to bacteria, archaea and viruses) [71]. SET domains (Figure 1h and SCOP superfamily: 82199) have a fold unique for SAM binding proteins – a substrate binding subdomain between two structural repeats, which may have evolved by duplication of 3-stranded unit with a generic ligand binding role [71,72]. Those repeats have a β-clip fold formed by double-stranded ribbons sharply bent in two places; the ribbon ends form incomplete barrel.
Similar duplication of a basic three-stranded unit containing the β-clip structural motif probably occurred also in related SAF and dUTPase superfamilies [72], which, however, tend to bind sugar and sugar derivatives [72]. There have been several other cases of adaptation of a generic ligand binding domain for SAM-binding, both in enzymes and in regulatory proteins without catalytic activity (see below).
SAM synthetase
S-adenosylmethionine synthetase (SAM synthetase, ATP:L-methionine S-adenosyltransferase, or MAT, EC: 2.5.1.6) is the main, or, possibly, the only enzyme of de novo SAM biosynthesis. SAM synthases from bacteria and eukaryotes are closely related at the sequence level and have very similar structures [73]. SAM synthases transiently interact with SAM prior to its release. The mechanism of reaction is thought to rely on conserved His14 (Figure 6), which acts as an acid to cleave the C5'-O5' bond of ATP, while simultaneously a change in the ribose ring conformation from C4'-exo to C3'-endo occurs, and the S of Met makes a nucleophilic attack on the C5' to form SAM [74].
thumbnailFigure 6. Multiple sequence alignment of SAM synthetases. Essential amino acids for the first (*) and the second substrate binding subunit are numbered as in gi:46015497 (PDB structure 1P7L and 1RG9). Aligned sequences represent protein from Archaea (gi:3334428 – M. jannaschii), Eukaryota (gi:400245 – H. sapiens, gi:6016553 – C. elegans), and Bacteria (gi:46015497 – E. coli, gi:15836994 – X. fastidiosa, gi:22095828 – F. nucleatum, gi:13357974 – U. parvum, gi:2500686 – M. pneumoniae, gi:21646472 – C. tepidum). Residues are highlighted according to the amino acid properties with designations as in Figure 2. Substrate binding is annotated below the alignment as follows: small letters – water mediated interactions; inverted colors – interactions with ligand from the second subunit of the homodimeric protein ; A/a – adenosyl moiety; R – ribosyl moiety, M/m – methionine moiety; (+) – Mg2+; K – K+; P – PPNP/Phosphate moiety. Consensus positions of the secondary structure elements are shown above the alignment. Numbers in parentheses indicate number of residues omitted for clarity.
The fold of bacto-eukaryal SAM synthetase is unique; each protein chain is based on a β-α-β-β-α-β module that folds into a wedge-like shape. A polypeptide chain consists of three such tandemly repeated modules, so that the complete SAM synthetase fold looks like a three-slice cream pie with topping made of β-sheets (Figure 1L). The active form of the enzyme appears to consist of two pies, with β-layers facing each other. Two SAM molecules are bound between the sheets of this dimer. In the E. coli enzyme, both subunits contribute many residues to SAM binding (Komoto et al. [74], Figure 6, and PDB structure: 1P7L and 1RG9) In particular, adenosine binds to Asp163, Arg229, Phe230 on one subunit and to Ser99 on another subunit, and interacts with many additional amino acids on both subunits via water-mediated hydrogen bond network. Methionine binds to Gln98 and Asp238 on one subunit, to Glu55 on another, and likewise makes many additional water molecule-mediated contacts. Bacto-eukaryal SAM synthetase is an evolutionary unique sequence and structural family. Even SAM decarboxylase, which is superficially similar in that it also sandwiches SAM between two β-sheets, has no detectable sequence or structure similarity to SAM synthetase.
SAM synthetases from Archaea have been isolated on the basis of their biochemical activity [75]. We performed sensitive searches of the conserved domain database, and found clear evidence for common ancestry of all SAM synthetases (Table 2). Multiple sequence alignment and secondary structure prediction indicate that archaeal enzymes share the same three-dimensional structure as their eukaryotic and bacterial homologs. All known SAM synthetases have conserved GHPD signature containing the main catalytic residue (His14 in E. coli gi:46015497) [75]. Despite high sequence divergence between archaeal and bacto-eukaryal enzymes, the complement of substrate binding residues is well-preserved (Figure 6). The apparent common origin of this unique enzyme in all major divisions of Life is of great interest for reconstruction of the repertoire of SAM-binding protein in the ancestral life forms (see below).
Table 1. Classes of SAM binding proteins
Table 2. Repertoire of SAM-binding proteins in the Last Universal Common Ancestor
SAM-binding modules derived from generic ligand-binding domains
ACC synthase
ACC synthase (S-adenosyl-L-methionine methylthioadenosine lyase, EC: 4.4.1.14, KOG0256) catalyses the rate-limiting step in biosynthesis of plant hormone ethylene by the α,γ-elimination of methylthioadenosine from SAM to produce 1-aminocyclopropane-1-carboxylate (ACC) [76]. ACC synthases require pyridoxal phosphate (PLP) for activity, and are related in sequence and structure to a large, diverse group of PLP-dependent transferases. The shared catalytic domain of this fold is of α/β/α type, with mixed central β-sheet of 7 strands (order 3245671), where strand 7 is antiparallel to the rest (SCOP fold: 53382; Figure 1j). Several residues are essential for the substrate binding (reviewed by Jakubowicz; numbered as in PDB structure 1B8G): Glu47 is responsible for putative ionic interaction with SAM; Ala46 and Arg407 interact with carboxypropyl moiety of SAM; Arg150 interacts with ribose moiety; and Ser18, Tyr19, Phe20, and Pro146 form hydrophobic pocket for the adenine ring of SAM [77]. Invariant Tyr85 is involved in the substrate recognition, and interacts with active-site Lys273 from the adjacent subunit (Lys273 forms a covalent Schiff base with PLP cofactor) [78,79].
Structure of ACC synthase is similar to other PLP-dependent transferases, such as transaminating aminotransferases, β-eliminating lyases, and cystathionine synthase. The only other enzyme in this group that binds SAM is 7,8-Diaminopelargonic acid (DAPA) synthase, which utilizes SAM in a different way than ACC synthase, namely as amino group donor in aminotransferase reaction. Both ACC synthase and DAPA synthase have similar active site residues involved in PLP cofactor binding. The interactions between SAM and DAPA synthase have not been studied in detail.
It is likely that ACC synthase and DAPA synthase evolved from other aminotransferases with different, perhaps broad, specificity, by accumulating changes in the ligand-binding region that increased its specificity towards SAM [80]. The evolutionary heritage of ACC synthase is manifest in the retained ability of the enzyme to catalyze slow transamination of substrates such as alanine [81]. Structural similarity between ACC-, DAPA-synthase and some other PLP-dependent enzymes (i.e. cystine C-S lyase – PDB structure 1ELQ; cystathionine β-lyase – PDB structure 1CL2) indicate that SAM binding in this case may have originated from ancestor with PLP-dependent binding of amino group of various sulfur containing amino acids or amino acid derivatives.
AHL synthase
Synthesis and detection of acyl-homoserine lactones (AHLs) enables many gram-negative bacteria to engage in quorum/diffusion sensing, an intercellular signaling mechanism that activates differentiation towards virulence and biofilm formation [82-84].
The AHL synthases (COG3916) catalyze acylation and lactonization of SAM, where the acyl group is provided by acylated acyl carrier protein (acyl-ACP) [85,86]. AHL synthases (SCOP family: 75508; Figure 1p) has acyl-CoA N-acyltransferase ("GNAT-like") fold: α/β/α sandwich with highly twisted β-sheet (SCOP fold: 55728). The conserved N-terminal residues: Arg23, Phe27, and Trp33 (numbered as in PDB structure 1RO5) form putative SAM binding pocket and undergo a dramatic conformational rearrangement upon acyl-ACP binding. This conformational change brings conserved residues of the putative SAM binding site in close proximity to the catalytic site [87]. Position of conserved β-bulge formed by Ser103 and Arg104 in β4 (numbered as in PDB structure 1RO5) distinguishes SAM-binding from other proteins with acyl-CoA-N-acyltransferase fold [86,87]. There is no detectable sequence or structural similarity between AHL synthases and other known SAM binding proteins, indicating independent origin of SAM binding in this fold. As with ACC synthase and DAPA synthase, the most likely mechanism of adaptation was by selecting relatively small changes in a generic ligand-binding region that increased relative affinity to SAM.
Met repressor
The E. coli MetJ repressor (Figure 1m; SCOP family: 100972; COG3060) uses SAM as a co-repressor to regulate the production of methionine. MetJ is a homodimeric, DNA-binding protein with ribbon-helix-helix fold. Co-repressor (SAM) binds to each monomer of the protein dimer at sites that lie on the opposite side of the protein from the DNA-binding motif. Binding of co-repressor affects DNA affinity, but apparently not specificity of MetJ [88-91]. Affinity of MetJ DNA binding is affected primarily by the positive charge associated with the ternary sulfur atom in co-repressor (SAM), which creates a region of positive electrostatic potential on the DNA binding surface overlapping the adjacent phosphodiester backbone in the region of the operator [92-94]. The SAM's adenine ring inserts itself deeply inside a hydrophobic pocket, consisted of side chains from both monomers. The positively charged sulfur of the SAM is greatly attracted by the net negatively charged C-terminal end of the β-helix, hence docking the SAM molecule in place. Electrostatic properties of SAM and its ability to serve as a regulatory feedback molecule in the common metabolic pathway of methionine synthesis probably played an important role in the emergence of this unique mode of SAM binding by MetJ.
MetJ is the only known SAM-binding representative of evolutionarily ancient ribbon-helix-helix (RHH) class of DNA-binding proteins [92,95]. Evolution of SAM cofactor binding in this protein was feasible because SAM adenosyl moiety fit into the cleft formed by both monomers, and its sulfonium center conformation was able to adapt to non-catalytic electrostatic interactions with MetJ repressor.
CBS
CBS-domains (COG0517) are widely distributed in all divisions of Life, in the form of fusions with various unrelated proteins, where they usually form tandem pairs. Binding of the adenosyl-containing molecules, such as ATP, AMP, and SAM by CBS-domains is important for their function as energy or redox status-sensing modules [96-98]. Some CBS-domains also binds single stranded nucleic acids [99].
In general, a tandem of CBS domains (encoded by ~120 aa) folds into one domain with a β-sandwich and 4 α-helices extending from one edge (Figure 1o). CBS domains within each pair are asymmetric. CBS-domain is common in multidomain proteins (i.e.: 15 in Bacteria and 9 in Archaea [100]) and is probably derived from generic small molecule-binding
